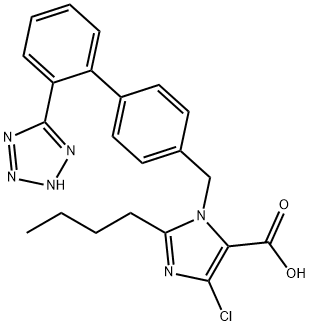
中文别名:
洛沙坦杂质14;5-羧酸氯沙坦;洛沙坦杂质;EXP-3174、5-羧酸洛沙坦;氯沙坦羧酸;洛沙坦-5-羧酸;5-羧酸洛沙坦;氯沙坦
英文名称:
2-BUTYL-4-CHLORO-1-[(2'-(1-H-TETRAZOL-5-YL)[1,1'-BIPHENYL]-4-YL)METHYL]-1-H-IMIDAZOLE-5-CARBOXYLIC ACID
英文别名:
LOSARTAN CARBOXY ACID;AKOS 91941;2-BUTYL-4-CHLORO-1-[(2'-(1-H-TETRAZOL-5-YL)[1,1'-BIPHENYL]-4-YL)METHYL]-1-H-IMIDAZOLE-5-CARBOXYLIC ACID;’-biphenyl)-4-yl)methyl)-;1h-imidazole-5-carboxylicacid,2-butyl-4-chloro-1-((2’-(14-tetrazol-5-yl)(1,1;E-3174;EXP-3174;E 3174;EXP 3174;E3174;EXP3174;2-Butyl-4-chloro-1-[[2'-(2H-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]-1H-imidazole-5-carboxylic acid;E-3174, EXP-3174
InChI:
InChI=1/C22H21ClN6O2/c1-2-3-8-18-24-20(23)19(22(30)31)29(18)13-14-9-11-15(12-10-14)16-6-4-5-7-17(16)21-25-27-28-26-21/h4-7,9-12H,2-3,8,13H2,1H3,(H,30,31)(H,25,26,27,28)
密度:
1.41±0.1 g/cm3(Predicted)
沸点:
707.8±70.0 °C(Predicted)
产品用途:
2-丁基-4-氯-5-羟甲基咪唑(2)与5-(4-溴甲基联苯-2-基)-1-三苯甲基-1H-四唑(3)反应生成(4);(4)先脱保护基生成2-丁基-4-氯-5-甲酰基-1-{[2′-(1-H-四唑-5-基)-联苯-4] -甲基}咪唑5);(5)经还原得氟沙坦(1)。

图1为氟沙坦的合成路线